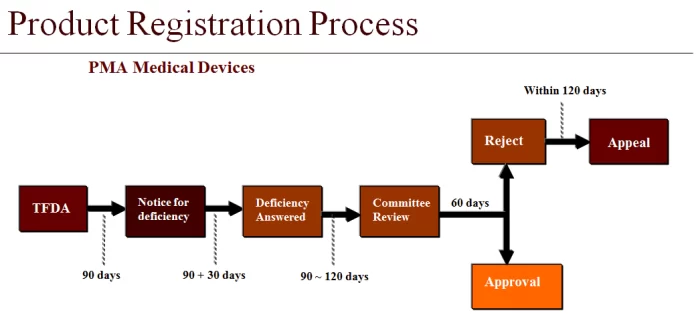
Navigating the intricacies of the medical device registration process in Taiwan is vital for any international business seeking to penetrate this growing market. Here, we will discuss an in-depth examination of the Taiwan medical device registration process and its pivotal role in ensuring public health and high-quality medical products.
Table of Contents
The Basis of Medical Device Registration
First, let’s clarify what we mean by medical device registration. It’s the regulatory process by which health authorities review the safety and effectiveness of a medical device before it can be sold or distributed. This critical step aims to safeguard public health and maintain the highest standards of medical products.
For international medical device companies, understanding this process is not just important—it’s essential. It can determine the speed at which products reach the market, their legal status, and the company’s overall reputation.
Tracing Back the Regulatory History
The medical device regulations in Taiwan have undergone substantial transformations to reach their current state. These changes have been driven by the country’s unwavering dedication to enhancing healthcare safety and maintaining high standards of quality.
The regulatory system in Taiwan was initially established with the primary objective of safeguarding public health. However, it has not remained stagnant; instead, it has experienced constant refinement and improvement over the years. Each modification has been strategically implemented to reinforce Taiwan’s commitment to ensuring the utmost quality of medical devices available in the market.
The motivation behind this evolving regulatory architecture arises from a profound sense of responsibility to protect the well-being of the public. Prioritizing the safety and efficacy of medical device products, Taiwan aims to cultivate an environment where patients can have confidence in the devices they rely on for their health and well-being.
Decoding the Registration Process
Now, let’s break down the medical device registration in Taiwan into its fundamental elements, providing an easy-to-follow guide on this important journey.
Taiwan Device Classification
In Taiwan, medical devices are classified based on their risk level, which ranges from Class I (lowest risk) to Class III (highest risk). This classification plays a significant role in determining the registration pathway. Understanding this risk-based classification system can help businesses prepare for the level of regulatory scrutiny their device will face and anticipate the time and resources needed for successful device registration.
Unraveling Pre-Market Registration
Before a medical device can hit the Taiwan market, it must go through pre-market registration. This involves the assessment of the device’s safety and efficacy, its intended use, and the benefits versus risks. Successfully completing pre-market registration is a significant milestone. It signifies the green light from regulatory authorities for a device to enter the Taiwan market, reinforcing its safety and effectiveness.
Quality System Documentation
Next in line is the preparation of Quality System Documentation. This is a detailed record that proves a medical device company adheres to the necessary quality management system (QMS) requirements.
The QMS encompasses a host of procedures that ensure consistent product quality and regulatory compliance. This documentation, a crucial part of the Taiwanese medical device approval, often includes design controls, supplier controls, and risk management procedures. The Taiwanese QSD system also looks at the factory’s manufacturing processes, equipment, etc.
Engaging Regulatory Authorities
Last but not least, Taiwanese regulatory authorities oversee the entire registration process. From reviewing applications to conducting inspections, these Taiwanese authorities play an integral role in upholding public safety and product quality. Engaging proactively with regulatory bodies can make the registration process smoother and more predictable. Cooperation is a two-way street and having open, constructive dialogues can facilitate understanding and compliance
Considering the Risks of Non-Compliance
Failure to adhere to the registration procedures could result in setbacks, including delayed Taiwan market entry, penalties, and reputational damage. These are not to be taken lightly. Non-compliance could result in your device being kept from the Taiwan market, significantly affecting your product launch timeline and profitability. Additionally, penalties for non-compliance can be severe, including fines or even complete product withdrawal. Moreover, neglecting the Taiwan registration process can tarnish a company’s reputation, impacting customer trust and potential business partnerships.
Guidance for International Businesses
In the complex world of Taiwan’s medical device regulations, international businesses can benefit from a step-by-step guide.
Preparing Documentation
Preparing comprehensive and high-quality documentation is vital for a successful registration process in Taiwan. Meeting regulatory standards is essential, but it is equally important to effectively showcase the safety and efficacy of your medical device through your documentation.
To streamline the registration process, it is crucial to familiarize yourself with the Taiwanese required documents and meticulously prepare them. This entails assembling technical specifications, clinical trial data, and other pertinent information, each playing a crucial role in supporting your application and substantiating the merits of your product
Ensuring Regulatory Compliance
Adhering to Taiwan’s regulatory standards is non-negotiable to ensure compliance. This entails meeting stringent quality system requirements, following labeling regulations precisely, and maintaining meticulous device traceability. To ensure continuous adherence to these Taiwanese standards, it is advisable to stay informed about any updates or revisions in the Taiwanese regulations. Regular internal audits should be conducted to assess and verify compliance, helping to identify any gaps or areas that require improvement.
Upholding Continuous Compliance
International medical device companies must uphold a commitment to maintaining continuous compliance as regulations can evolve over time. This entails closely monitoring any changes in the Taiwanese regulatory landscape, proactively adjusting practices, and promptly implementing necessary modifications to ensure ongoing adherence. Embracing a proactive and adaptable approach to compliance, businesses can navigate the ever-changing medical device regulatory environment in Taiwan successfully.
The Taiwan medical device registration process may seem daunting, but with understanding and preparation, it can be navigated successfully. Prioritizing compliance, businesses can ensure a smoother path to market entry, ultimately serving healthcare providers and patients with high-quality, safe, and effective medical devices.